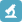光聚合生物材料和基于光的3D打印策略在生物医学中的应用
发布时间:2024-06-19 分享至:
【引言】
自从增材制造(通常称为3D打印)技术问世以来,这项技术彻底改变了生物制造领域,并推动了组织工程和再生医学领域的许多关键性进展。具体来说,与传统的2D技术相比,现在已经有了较多的文献证明,刚性单层培养系统不能很好地复原天然环境中固有的复杂性,因此,在这种2D条件下生长的细胞很难反映体内功能、表现型、形态和分化潜能,从而受到这种称之为细胞外基质(ECM)的高度影响。因此,3D细胞培养系统在组织工程和再生医学领域获得了广泛的吸引力。同时为了正确地模拟3D ECM环境,需要一种能够精确控制材料在3D空间中的力学、物理和粘弹性性能的制造方法。从***新的3D打印技术进展表明,它们有望满足这些要求。3D打印机所提供的控制水平已使得在生产与生理相关的仿生组织和器官替代品方面取得许多显著进展,如药物测试,阐明生物机制,疾病模型,翻译医学和外科植入物等。事实上,自Charles Hull博士***将立体平版印刷(SLA)引入世界之后,许多3D打印技术也在短时间内被开发出来。然而,相应的3D打印材料并没有被发展起来,这也是一段时间以来制约该领域发展的瓶颈。在***近的十年里,研究者才逐渐认识得到发展3D打印材料的重要性,从而***大化挖掘3D打印技术真正的潜力。
近日,美国加州大学圣地亚哥分校(UCSD)纳米工程系陈绍琛教授(Shaochen Chen)(通讯作者)回顾了适合于光基3D打印技术的生物材料的发展,及其重点在生物打印方面的应用。首先,作者介绍了光固化生物材料中光聚合反应的基本原理和机理,总结了常用的光抑制和光不稳定的化学物质来控制聚合动力学。随后,讨论了目前用于光基3D打印的光聚合天然、合成和复合生物材料的文献,以及它们在组织工程和再生医学的应用。***后,作者回顾了***近从串行到平面再到体积构建的光基3D打印技术的进展和演变,并讨论了提高打印分辨率和质量控制的策略,以标准化未来的打印优化方法。总体而言,扩大和发展新型光固化生物材料将有助于促进和扩大光基3D打印技术的用途。相关研究成果以“Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications”为题发表在Chem. Rev.上。
【图文导读】
图一、光基3D打印技术在组织工程和再生医学应用中的生物材料选择标准概述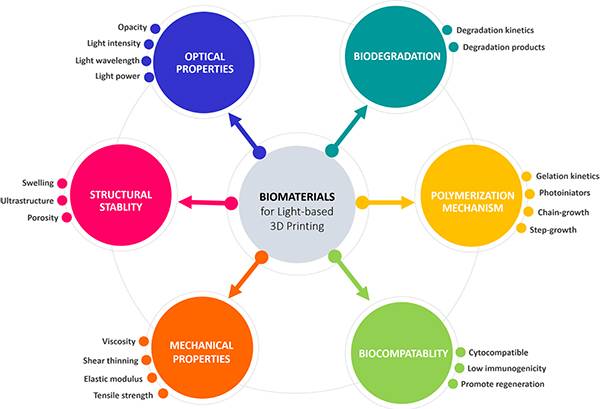
图二、自由基引发硫醇−烯化学反应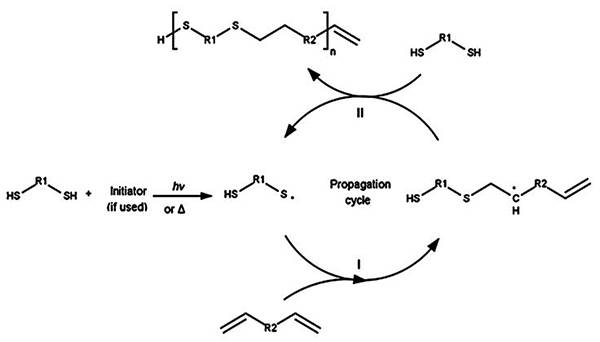
图三、烯烃基团选择对硫醇−烯反应动力学的影响 (A)硫醇−烯反应动力学的理论计算取决于所选择的烯烃基团的反应性;
(A)硫醇−烯反应动力学的理论计算取决于所选择的烯烃基团的反应性;
(B)基于理论动力学模型的烯烃基团反应性递降。
图四、取决于不同交联机理和由此产生的不均匀程度的水凝胶网络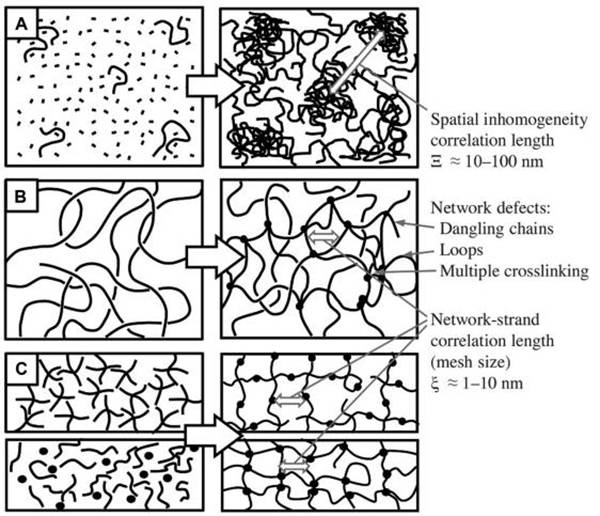 (A)单体和交联剂的自由基链生长聚合导致网络结构中的空间不均匀性;
(A)单体和交联剂的自由基链生长聚合导致网络结构中的空间不均匀性;
(B)聚合物链的官能团在半静态溶液中通过交联形成网络,导致局部不均匀
(C)聚合形成一个基本有序、均匀的网络。
图五、邻硝基苄基(R1=H)和硝基苯基(R1=甲基)的光解机理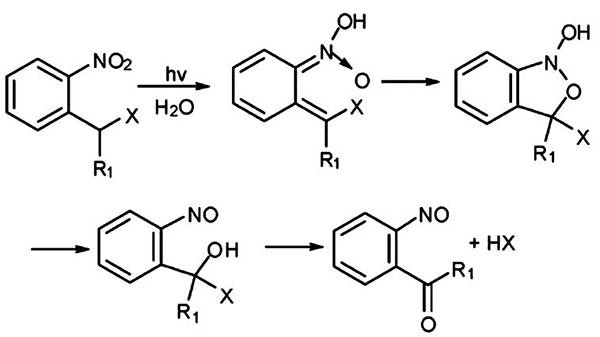 图六、生物材料的3D打印技术
图六、生物材料的3D打印技术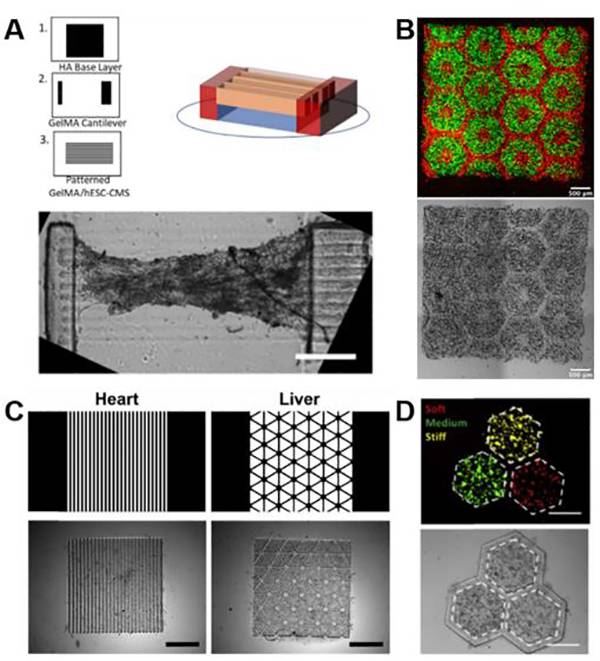 (A)使用GelMA打印的悬臂式心脏组织的示意图和图像;
(A)使用GelMA打印的悬臂式心脏组织的示意图和图像;
(B)使用GelMA和GM-HA生物模拟打印的多细胞肝组织用于药物试验的荧光和亮场图像;
(C)使用组织特异性dECM生物墨水模拟心脏和肝脏组织的设计和图像;
(D)使用dECM生物墨水打印的肝癌模型荧光及图像。
图七、用于细胞生物学的各种3D打印PEG基水凝胶结构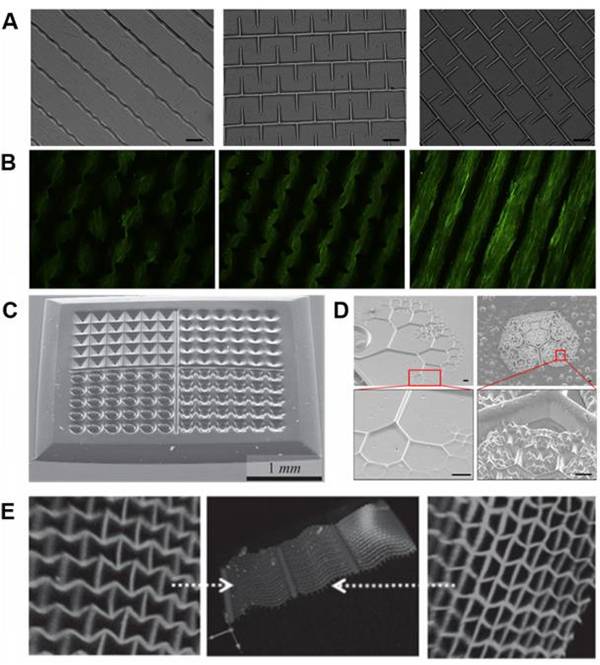 (A)3D打印的PEGDA图像;
(A)3D打印的PEGDA图像;
(B) 三种PEGDA模式的细胞排列和肌形成;
(C)3D印制中各种形状的微孔,用于多细胞球体和胚状体培养;
(D)研究细胞组织行为的自然激发分形模式;
(E)具有微尺度单位和正负泊松比的3D打印网络结构
图八、用于组织工程和再生医学的各种3D打印PEG基水凝胶结构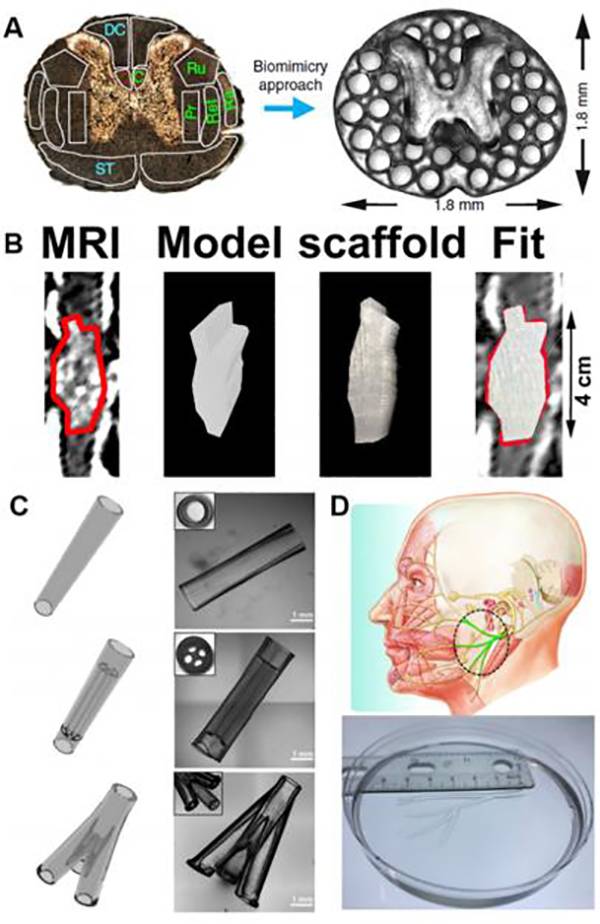 (A)3D打印仿生脊髓支架;
(A)3D打印仿生脊髓支架;
(B)基于人体脊髓损伤MRI的3D打印脊髓支架;
(C)各种用于周围神经再生的3D打印神经引导导管;
(D)人面部大小NGC的3D打印。
图九、3D打印的NOr-PGS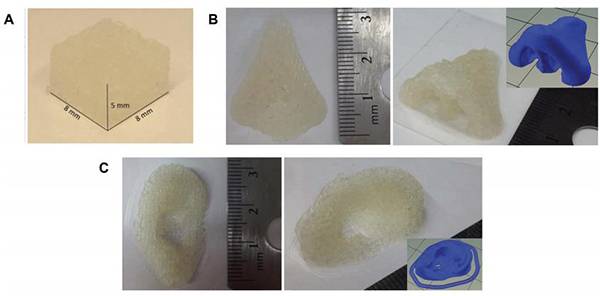
将Nor-PGS3D打印为(A)立方体,(B)鼻子形和(C)耳朵形结构
图十、聚氨酯的聚合机理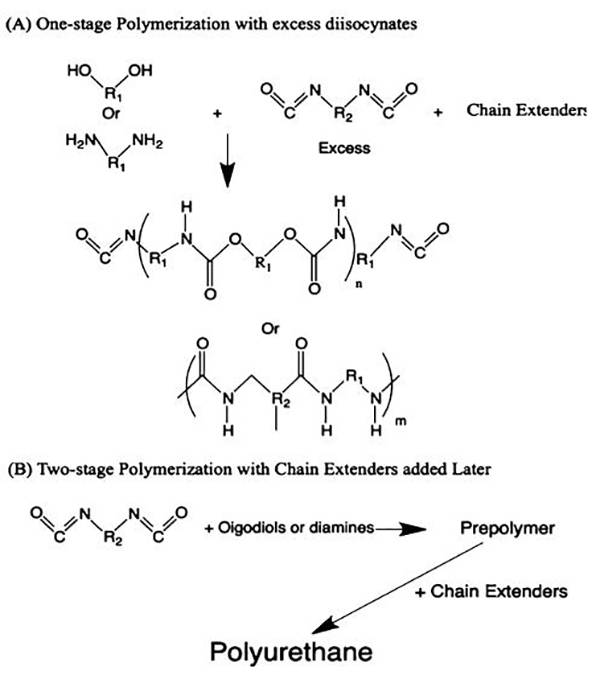 (A)多元醇/多胺和扩链剂与过量二异氰酸酯之间的一级聚合;
(A)多元醇/多胺和扩链剂与过量二异氰酸酯之间的一级聚合;
(B)多元醇/多胺与二异氰酸酯之间的两级聚合。
图十一、大规模聚氨酯生产中常用的二异氰酸酯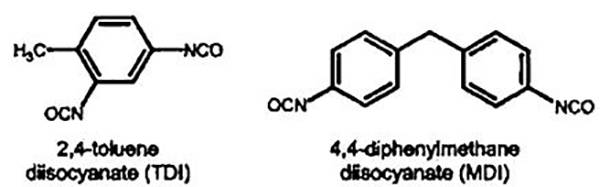
图十二、聚氨酯生产中常用的低聚物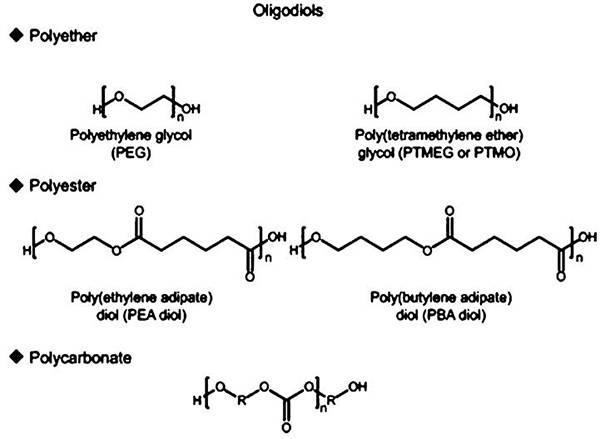
图十三、热塑性聚氨酯和热固性聚氨酯聚合物链结构差异的示意图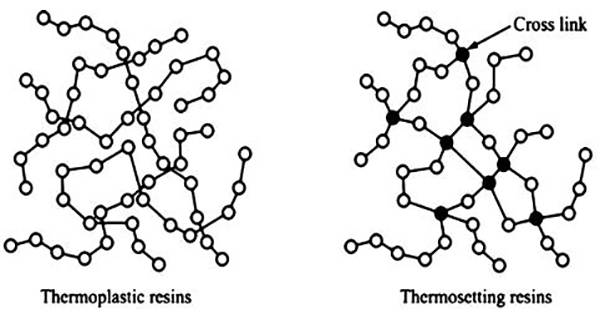
图十四、在PU中硬、软段分布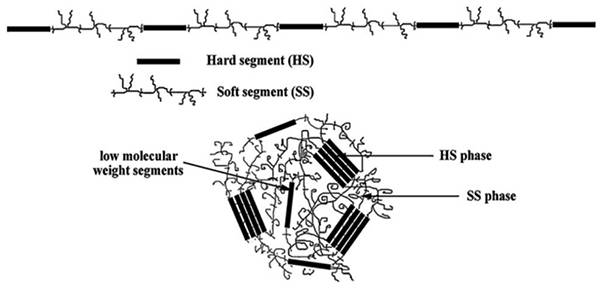
图十五、可用于形成纳米复合水凝胶的不同类型纳米材料的示意图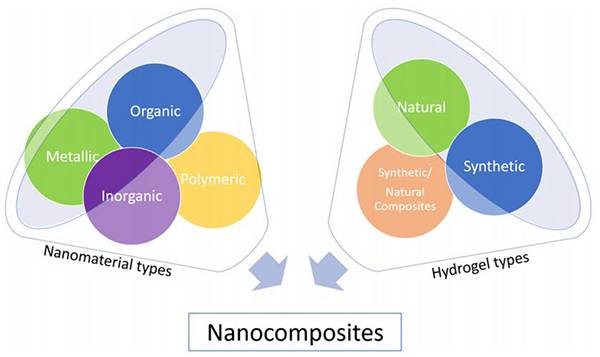 图十六、CNT/GelMA的3D打印
图十六、CNT/GelMA的3D打印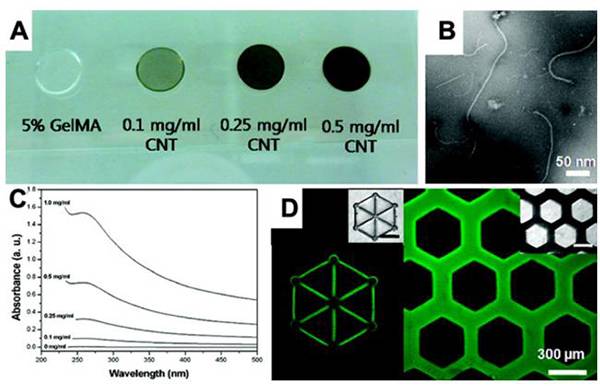 (A)CNT/GelMA预聚物溶液的光学图像;
(A)CNT/GelMA预聚物溶液的光学图像;
(B)0.5 mg/mL CNT/GelMA预聚物溶液的高分辨率TEM图像;
(C)预聚物溶液的UV−vis吸附光谱;
(D)CNT/GelMA水凝胶的荧光图像。
图十七、微形鱼图像的3D打印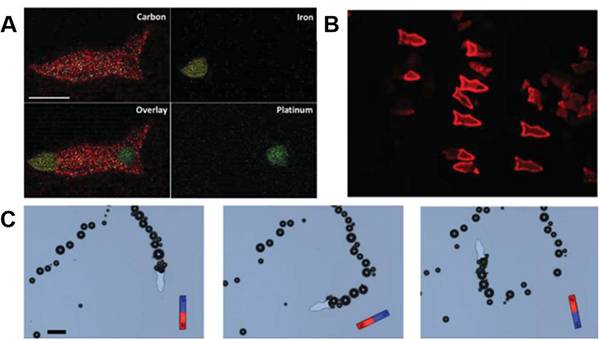 (A)定位于头部、尾部和身体的3D微鱼的不同纳米粒子的能量色散X射线;
(A)定位于头部、尾部和身体的3D微鱼的不同纳米粒子的能量色散X射线;
(B)3D打印的蜂胶溶液微鱼的荧光图像;
(C)微鱼在磁力引导下不同时间的图像。
图十八、羟磷灰石(HA)的3D打印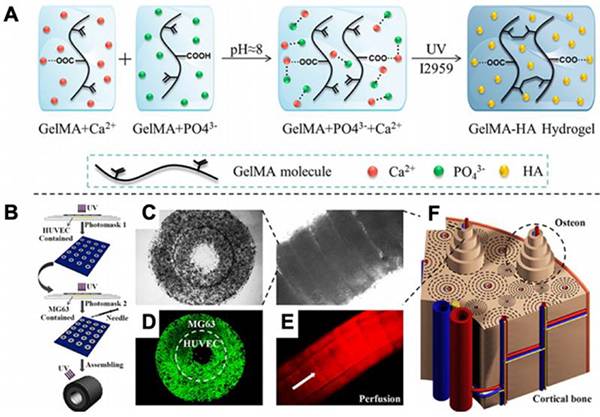 (A)GelMA网络中羟磷灰石(HA)形成机理的示意图;
(A)GelMA网络中羟磷灰石(HA)形成机理的示意图;
(B)打印装置原理图;
(C)3D打印样品的表征;
(D)结构中细胞的共焦图像;
(E)若丹明(红色)灌注管的荧光图像
(F)3D打印皮质骨示意图。
图十九、3D打印肝脏解毒装置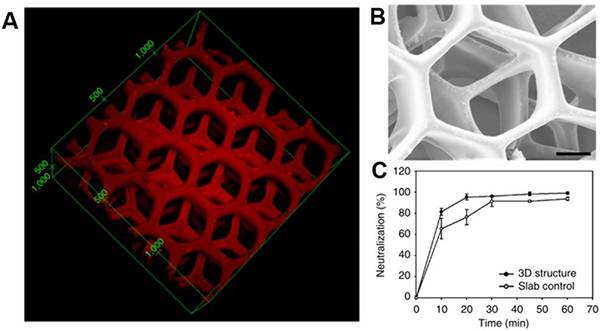 (A) 聚二乙炔纳米粒子包裹在PEGDA中的3D肝脏驱动解毒装置的荧光图像;
(A) 聚二乙炔纳米粒子包裹在PEGDA中的3D肝脏驱动解毒装置的荧光图像;
(B)这种解毒装置的SEM图像;
(C)肝脏驱动的解毒装置显示更高的中和效率。
图二十、基于光的3D打印模式的分类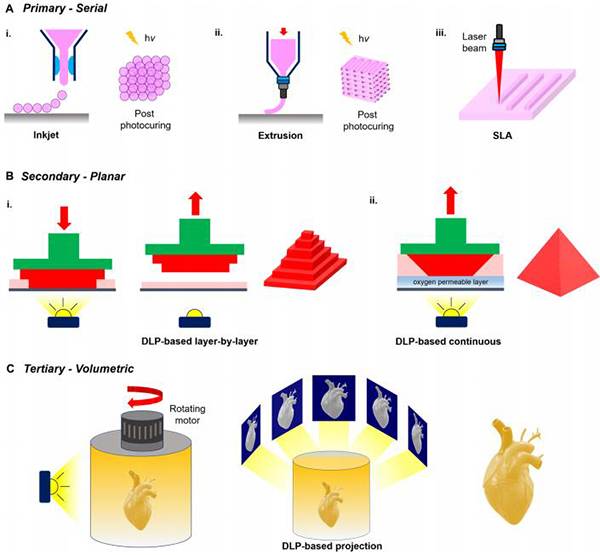 (A)以逐点或逐行方式连续沉积的生物材料;
(A)以逐点或逐行方式连续沉积的生物材料;
(B)基于数字光处理(DLP)的平面构建模式投影到生物材料;
(C)基于DLP的模式投影的体积构建投影到生物材料。
【小结】
总之,多年来3D打印技术已迅速发展成为在制造生物医学应用的高度复杂结构的先进系统。这种新型的制造方法已经用于开发新型骨架、组织和器官替代品以及医学植入物,从而实现在传统生物制造中无法实现的研究方法。同时本文中还强调了光基3D打印机技术在发展过程中的重要作用,即基于光的3D打印技术可以分为从串行到平面到体积构建的分层打印模式,同时将重点放置于后两种模式上,其通过DLP的技术实现,这主要是由于其优越的微米级分辨率、 以秒到分钟的顺序快速制造速度和可扩展性。此外,识别和理解每个参数的影响对于改进的下一代3D打印技术的设计和工程是非常有价值的。
文献链接:“Photopolymerizable Biomaterials and Light-Based 3D Printing Strategies for Biomedical Applications”(Chem. Rev.,2020,DOI: 10.1021/acs.chemrev.9b00810)
本文由CYM编译供稿。
作者简介
Shaochen Chen, PhD
Professor and Chair of NanoEngineering Department
University of California, San Diego
Research: Dr. Chen is a pioneer in 3D printing and bioprinting with over 200 peer-reviewed publications. He first initiated a scanningless 3D printing technique termed "micro-stereolithography (µSL)" for projection printing of biomaterials in 2006. Building upon his µSL technique, he invented a dynamic optical stereolithography method (DOPsL) in 2012 (Advanced Materials, 2012). Compared to traditional nozzle-based 3D printing, DOPsL enables 3D printing that is 3,000 times faster in printing speed and 100 times finer in printing resolution (Nature Communications, 2014). He has continued to advance this field by developing a microscale continuous optical bioprinting (µCOB) method for the rapid 3D bioprinting of functional tissues models in mere seconds. Using human induced pluripotent stem cells, he successfully bioprinted functional liver tissues that enable disease modeling and drug screening (PNAS, 2016). Furthermore, by integrating neuron stem cells within a 3D printed biomimetic scaffold, his team has succeeded in the repair of a severely damaged spinal cord in rats to result in significant functional recovery (Nature Medicine, 2019). His ground-breaking work has been reported by The Washington Post, The Wall Street Journal, Forbes, and Yahoo News.
His pioneering work in micro and nanoscale 3D printing and bioprinting established the foundation for the emerging field of biofabrication for tissue engineering and regenerative medicine applications. He founded a startup company, Allegro 3D to commercialize his bioprinting techniques. It is providing transformative solutions to organ/tissue repair and regeneration, accelerating drug toxicity and efficacy testing, and advancing human diseases modeling.
Dr. Chen has received numerous awards, including the NSF CAREER award, ONR Young Investigator award, and NIH Edward Nagy New Investigator Award. In 2017, he received the Milton C. Shaw Manufacturing Research Medal from ASME for his seminal work in 3D printing, bioprinting, and nanomanufacturing. This is the highest award given by ASME to recognize original manufacturing research in the field. Dr. Chen is a Fellow of major societies, including the American Association for the Advancement of Science (AAAS), American Institute for Medical and Biological Engineering (AIMBE), American Society of Mechanical Engineers (ASME), International Society for Optics and Photonics (SPIE), and International Society for Nanomanufacturing (ISNM).
Representative Publications (out of 203 peer-reviewed papers)
Lu and S. C. Chen*, “Micro and Nano-fabrication of Biodegradable Polymers for Drug Delivery”, Advanced Drug Delivery Reviews, Vol. 56, pp. 1621-1633, 2004.
Lu, G. Mapili, G. Suhali, S. C. Chen*, K. Roy*, “A Digital Micro-mirror Device-based System for the Microfabrication of Complex, Spatially Patterned Tissue Engineering Scaffolds”, Journal of Biomedical Materials Research A, Vol. 77A (2), pp 396-405, 2006.
P. Zhang,X. Qu, P. Soman, K. C. Hribar, J. W. Lee, S. C. Chen*, and S. He, “Rapid Fabrication of Complex 3D Extracellular Microenvironments by Dynamic Optical Projection Stereolithography”, Advanced Materials, Vol. 24 (no. 31), pp. 4266-4270, 2012.
Zhu, J. Li, Y. Leong, I. Rozen, X. Qu, R. Dong, Z. Wu, W. Gao, P. H. Chung, J. Wang*, and S. C. Chen*,“3D Printed Artificial Micro-Fish”, Advanced Materials, 27, pp. 4411–4417, 2015.
Ma, X. Qu, W. Zhu, Y.-S. Li, S. Yuan, H. Zhang, J. Liu, P. Wang, C. S. Lai, F. Zanella, G.-S. Feng, F. Sheikh, S. Chien*, S. C. Chen*, “Deterministically Patterned Biomimetic Human iPSC-derived Hepatic Model via Rapid 3D Bioprinting”, Proceedings of the National Academy of Sciences (PNAS), Vol. 113 (no. 8), pp. 2206-2211, 2016.
Highlighted in Nature Reviews Gastroenterology & Hepatology, Feb 24, 2016.
Zhu, X. Qu, J. Zhu, X. Ma, S. Patel, J. Liu, P. Wang, C. S. Lai, M. Gou, Y. Xu, K. Zhang, S. C. Chen*, “Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture”, Biomaterials, Vol. 124, pp. 106-115, 2017.
Zhu+, K. R. Tringale+, S. A. Woller, S. You, S. Johnson, H. Shen, J. Schimelman, M. Whitney, J. Steinauer, W. Xu, T. L. Yaksh, Q. T. Nguyen*, S. C. Chen*, “Rapid Continuous 3D Printing of Customizable Peripheral Nerve Guidance Conduits”, Materials Today, Vol. 21 (9), pp. 951-959, 2018.
Ma, C. Yu, P. Wang, W. Xu, X. Wan, C. S. E. Lai, J. Liu, A. Koroleva-Maharajh, S. C. Chen*, “Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture”, Biomaterials,Vol. 185, pp. 310-321, 2018, DOI: 10.1016/j.biomaterials.2018.09.026
Koffler+, W. Zhu+, X. Qu, O. Platoshyn, J. Dulin, J. Brock, L. Graham, P. Lu, J. Sakamoto, M. Marsala, S.C. Chen*, M. H. Tuszynski*, “Biomimetic 3D-Printed Scaffolds for Spinal Cord Injury”, Nature Medicine, Vol. 25, pp. 263-269, 2019.
Highlighted in Nature Reviews Neuroscience, Jan. 29, 2019, reported by NIH Director’s Blog on June 6, 2019.
Tang, Q. Xie*, R. C. Gimple, Z. Zhong, T. Tam, J. Tian, R. L. Kidwell, Q. Wu, B. C. Prager, Z. Qiu, A. Yu, Z. Zhu, P. Mesci, H. Jing, J. Schimelman, P. Wang, D. Lee, M. H. Lorenzini, D. Dixit, L. Zhao, S. Bhargava, T. E. Miller, X. Wan, J. Tang, B. Sun, B. F. Cravatt, A. R. Muotri, S.C. Chen*, J. N. Rich*, “Three-dimensional bioprinting enables creation of tissue-informed glioblastoma microenvironments for modeling complex cellular interactions”, Cell Research, in press, 2020
Wangpraseurt*, S. You, F. Azam, G. Jacucci, O. Gaidarenko, M. Hildebrand, M. Kühl, A. G. Smith, M.P. Davey, A. Smith, D. D. Deheyn, S. C. Chen*, S. Vignolini*,“3D Printed Bionic Corals”, Nature Communications, Vol. 11, 1748 (1-8), 2020.